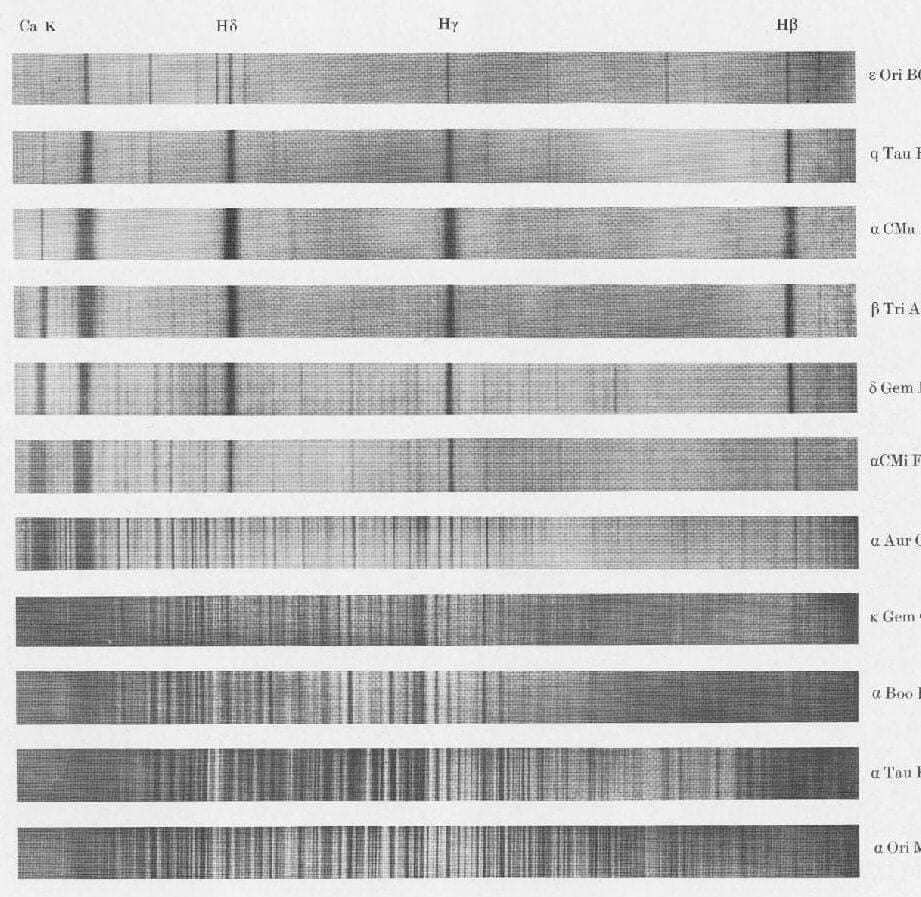

Despite their varying sizes, all of these stars initially possessed similar compositions.
The composition of stars dictates their characteristics and destinies, ranging from their hue and luminosity to their lifespans. Additionally, the composition of a star influences the entirety of its formation process, as well as the creation of its planetary system – and even our own solar system.
A universal standard
Every star in its initial stage, whether it is a massive giant like UY Shield or a yellow dwarf like our Sun, consists of approximately equal amounts of the same elements. This includes 73% hydrogen, 25% helium, and an additional 2% of heavy atoms. The composition of the universe after the Big Bang was almost identical, except for the 2% of heavy elements that were created through the explosions of the first stars in the cosmos. These explosions were so powerful that they surpassed the size of today’s galaxies.
However, what makes the stars so unique? The answer lies in the additional 2 percent of their composition. This factor is not the only one, as the mass of the star also plays a significant role. The fate of a star is determined by the gravitational pressure it experiences – some stars, like Canopus, will burn out in a few hundred million years, while others, like the Sun, will continue to shine for billions of years. However, the additional matter in a star’s composition can override all other factors.
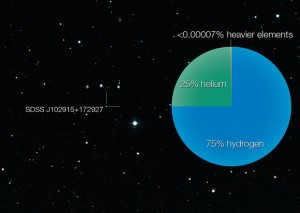
The chemical makeup of the star SDSS J102915 +172927 is indistinguishable from that of the initial stars that arose following the occurrence of the Big Bang.
Exploring the star’s interior
However, how could such a minuscule portion of a star’s composition significantly impact its operation? For a human, whose body comprises an average of 70% water, a mere 2% loss of fluid is not catastrophic – it only results in intense thirst and does not produce irreversible alterations in the body. Nevertheless, the cosmos is incredibly sensitive to even the slightest modifications – had the 50th portion of our Sun’s composition been marginally different, life may not have developed within the solar system.
During the process of star formation from dispersed material, a similar occurrence takes place. According to the traditional model of star structure, the core of the star consists of helium, while the surrounding envelope is primarily composed of hydrogen. Once the mass of helium surpasses a certain threshold, the gravitational forces become so intense that they compress the core, initiating a thermonuclear reaction within the layers that lie between the helium and hydrogen in the core.
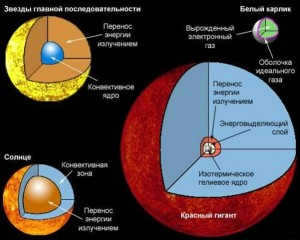
At this point, the star is ignited – still in its infancy, surrounded by hydrogen clouds that will eventually settle on its surface. The star’s luminosity plays a crucial role in its existence – it is the particles attempting to escape from the core following the thermonuclear reaction that prevent the luminosity from instantly collapsing into a neutron star or black hole. Additionally, ordinary convection exerts its force, causing matter to move under the influence of temperature – hydrogen atoms, ionized by the intense heat at the core, rise to the upper layers of the star, effectively mixing the matter within it.
Therefore, the presence of 2% heavy matter in the composition of a star is crucial. Elements like carbon, oxygen, and metals, which are heavier than helium, will always be drawn to the center of the star’s core. This lowers the threshold for triggering a thermonuclear reaction, causing the core to ignite faster. However, this also means that the star will emit less energy. The size of the hydrogen combustion epicenter will be smaller if the core is made up of pure helium.
Does the Sun have good luck?
Therefore, approximately 4.5 billion years ago, when the Sun initially transformed into a fully developed star, it possessed the same composition as the rest of the Universe – 75% hydrogen, 25% helium, and a small fraction of metallic impurities. The unique combination of these additives rendered the Sun’s energy suitable for sustaining life within its system.
When we talk about metals, we are not only referring to nickel, iron, or gold – astronomers classify any element other than hydrogen and helium as a metal. The nebula from which the Sun is believed to have originated contained a significant amount of heavy elements – the remnants of supernova stars that produced the heavy elements in the cosmos. Stars with similar nucleation conditions to the Sun are categorized as population I stars. These celestial bodies make up the majority of our galaxy.
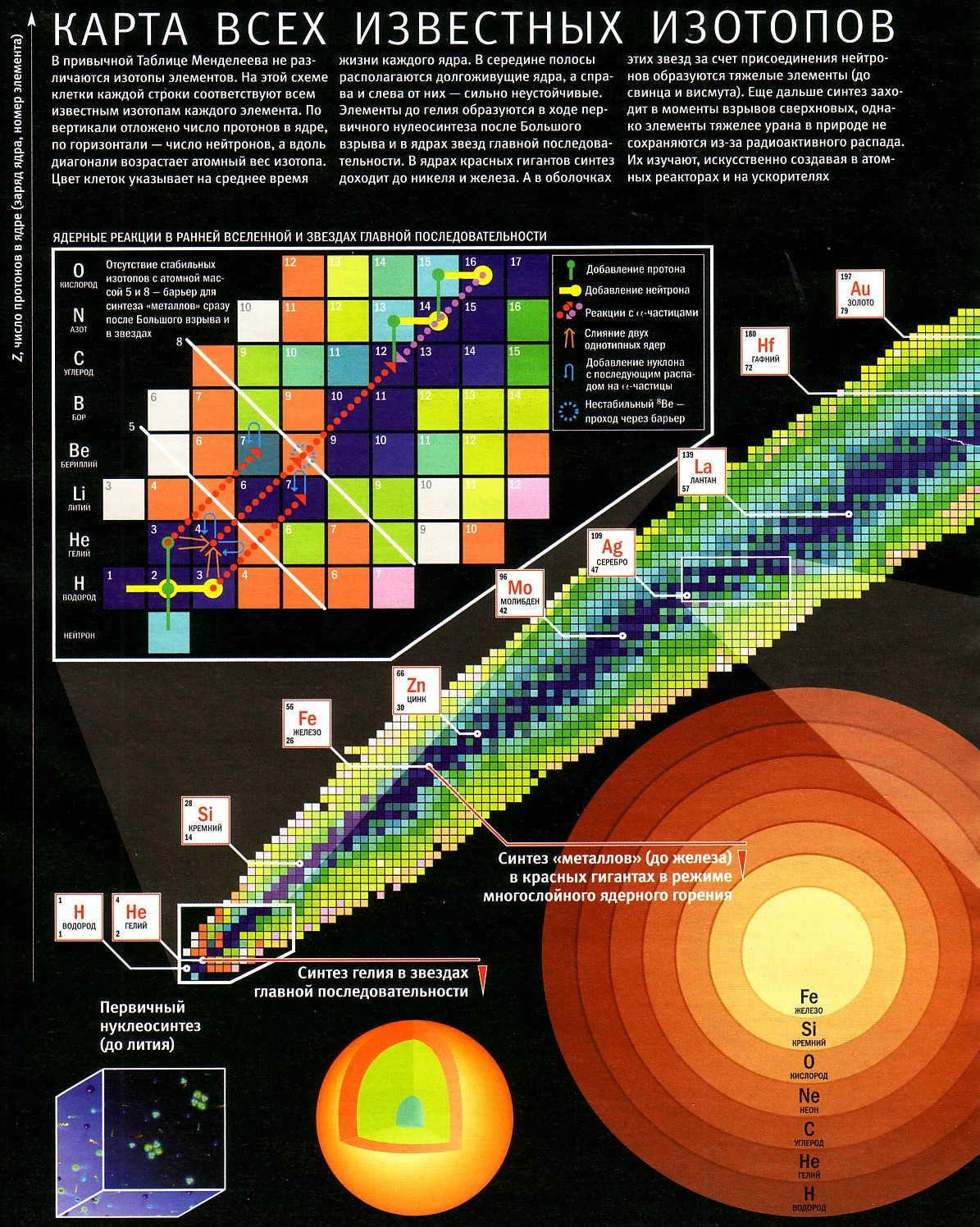
A diagram displaying the elements produced from nuclear reactions within stars. Click to enlarge.
It is already understood that the presence of 2% of metals in the Sun’s composition leads to a slower rate of burning. This characteristic ensures both a prolonged lifespan for the star and a consistent supply of energy, which are important factors for the development of life on Earth. Additionally, the early initiation of thermonuclear reactions prevented the absorption of all heavy elements by the young Sun. As a result, these elements were able to form and eventually contribute to the formation of the planets that exist today.
The star’s maturation and alteration in its constitution
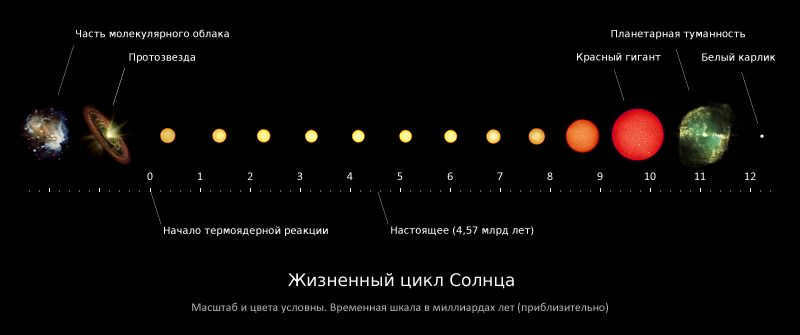
However, time does not remain stagnant – and the composition of stars gradually changes due to thermonuclear reactions. The primary and simplest fusion reaction that occurs in the majority of stars in the Universe, including our Sun, is known as the proton-proton cycle. During this process, four hydrogen atoms combine to create one helium atom and a tremendous amount of energy – up to 98% of the total energy emitted by the star. This phenomenon is also referred to as the “burning” of hydrogen: the Sun consumes approximately 4 million tons of hydrogen every second.
What happens to a star’s composition as it gets older? This can be understood from the information we already know about stars in the article. Let’s use our Sun as an example: the amount of helium in its core will increase, leading to an expansion of the core. As a result, the region of thermonuclear reactions will also expand, resulting in an increase in both the brightness and temperature of the Sun. After 1 billion years (when the Sun is 5.6 billion years old), the star’s energy will increase by 10%. By the time the Sun is 8 billion years old (3 billion years from now), its radiation will be 140% of its current levels – this will cause such significant changes on Earth that it will resemble Venus.
Resources on the subject
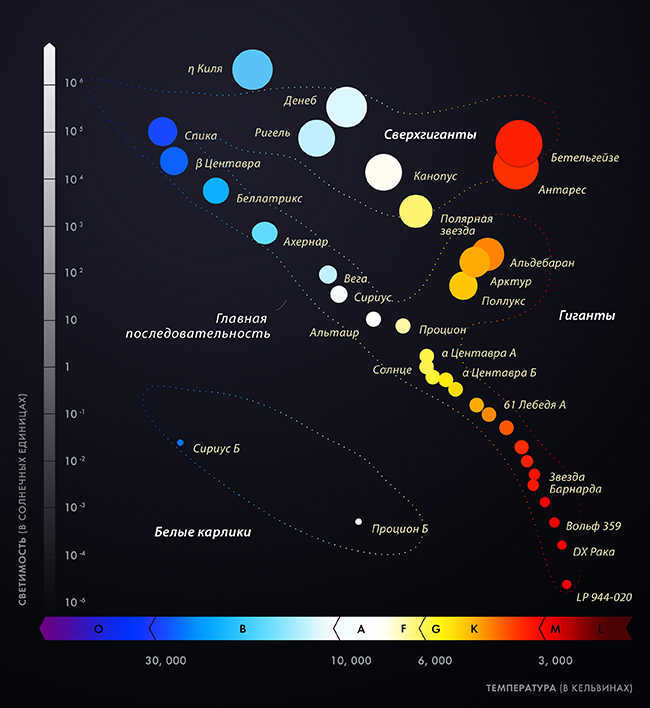
The enhancement in the intensity of the proton-proton collision will have a significant impact on the structure of the star – hydrogen, which has been relatively unaffected since its formation, will commence combustion at a much more rapid rate. The equilibrium between the Sun’s outer layer and its inner core will be disrupted – the hydrogen layer will expand while the helium core, conversely, will contract. When the star reaches an age of 11 billion years, the radiation pressure from its core will become weaker than the gravitational force compressing it – at this point, it will be the increasing compression that provides the core with its warmth.
There will be notable alterations in the makeup of the star within a billion years, as the temperature and compression of the Sun’s core will escalate to such an extent that the subsequent phase of thermonuclear fusion – helium “burning” – will take place. As a consequence of this reaction, helium nuclei will collide and transform into an unstable state of beryllium, subsequently forming carbon and oxygen. The magnitude of this reaction is immensely substantial – upon ignition of the remaining pockets of helium, the Sun will radiate approximately 5,200 times more brightly than its current luminosity!


The Red Giant Sun as seen from Earth in an artistic interpretation.
Throughout these processes, the Sun’s core will continue to increase in temperature, and the outer layer will expand up to the Earth’s orbit and experience a significant decrease in temperature – as the area of radiation expands, the body loses more energy. The mass of the star will also be affected: solar winds will carry away the remaining helium, hydrogen, and newly formed carbon and oxygen into outer space. Thus, our Sun will transform into a red giant. The star will reach its final stage when the outer layer is completely depleted, leaving behind a dense, hot, and small core – a white dwarf. Over billions of years, it will gradually cool down.
The development of stellar composition in stars distinct from the Sun
Upon reaching the helium ignition phase, the thermonuclear reactions within a star comparable in size to the Sun reach their conclusion. In the case of smaller stars, their mass is insufficient to initiate the fusion of newly formed carbon and oxygen – a celestial body must possess a mass at least five times greater than that of the Sun in order for carbon to undergo nuclear metamorphosis.
The sequence of changes in massive stars is much more extensive: it extends until it reaches iron. The heavier components are produced. These stars have no possibility of reverting – they will detonate in a supernova, resulting in the formation of a black hole or a neutron star. The latter does not consist of the conventional matter we are familiar with – the star is filled with an incredibly dense superfluid that causes the protons and electrons within it to combine into uncharged particles, neutrons. A small container of highly concentrated star substance would weigh hundreds of millions of tons.
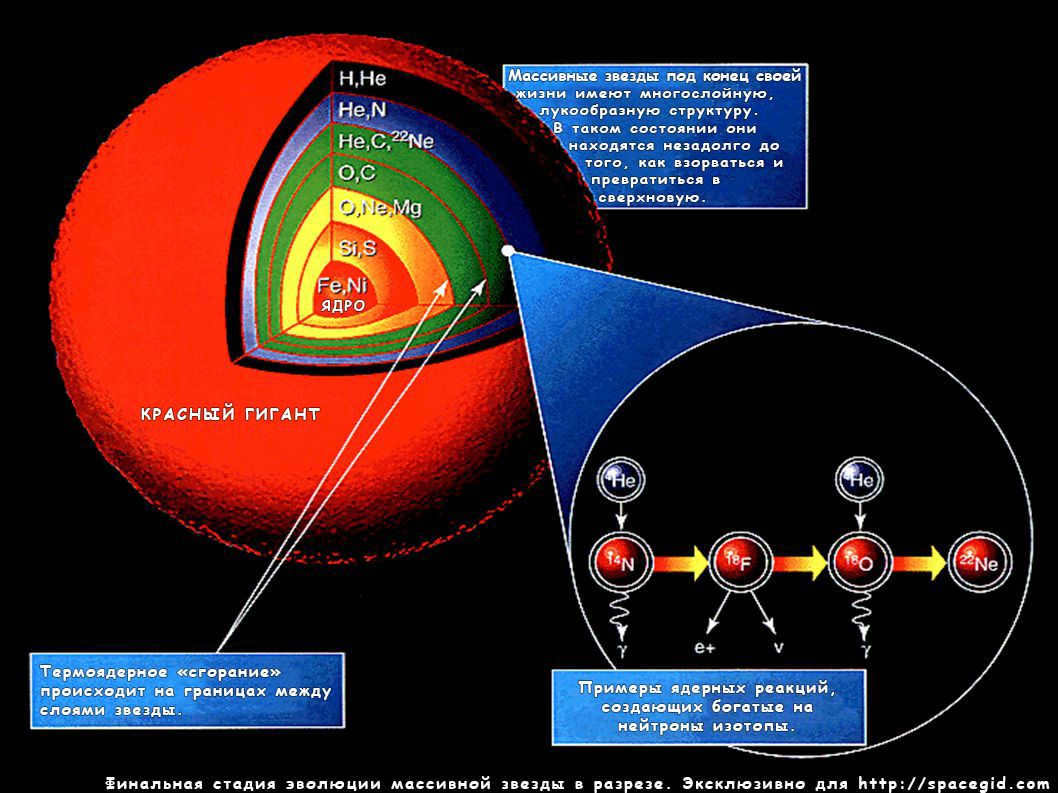
The final stage in the life cycle of a massive star involves a fascinating process. While carbon and oxygen coexist within the star, they undergo fusion reactions that result in the creation of different elements at varying depths within the star. Carbon, for instance, gives rise to lighter elements such as neon, sodium, and magnesium. On the other hand, oxygen leads to the production of heavier non-metals like sulfur or phosphorus, as well as loose metals like aluminum. Additionally, these elements, along with nitrogen, play a crucial role in the CNO cycle of hydrogen fusion, which is the primary thermonuclear process in large Main Sequence stars. By acting as catalysts, they facilitate the “burning” of hydrogen, allowing it to occur with less gravitational compression.

Emission spectra of various light sources
It’s worth noting that an intriguing piece of information is that when one gram of hydrogen undergoes fusion, it produces a staggering 98 thousand kilowatt-hours of energy. To put this into perspective, one gram of uranium in a nuclear reactor generates 22 thousand kWh, while the conventional combustion of hydrogen yields a mere 4.4 watt-hours.
What is the process of discovering the composition of stars?
Discovering the composition of stars has been a complex and intriguing endeavor. Throughout history, scientists have made great strides in understanding various aspects of stars, but determining their composition has proven to be particularly challenging. It wasn’t until the 18th century that philosopher Immanuel Kant proposed a theory about the origin of stars, shedding some light on their formation. However, it took much longer for researchers to accurately determine the materials that make up stars.
In the middle of the 19th century, scientists finally managed to unveil the enigma thanks to the breakthrough invention of spectral analysis technique. The astonishing revelation was that every light source possesses its own distinct emitted spectrum, which is directly determined by its composition – certain materials absorb specific lines from the spectrum while allowing others to pass through. By harnessing the power of spectral analysis, astronomers have immensely broadened the realms of human understanding.
An o-class (50000-10000C) star’s atmosphere can be analyzed spectrally, revealing hydrogen and helium ionization lines. It has also been observed that metal ions of k-class (5000C) stars have already been discovered.
Hydrogen and helium lines dominate the list of stars belonging to the first four classes. However, as the temperature decreases, lines of other elements begin to appear, although they still remain relatively simple. These elements include oxides like zirconium, titanium (class M), and solar radicals.
The outer layer of a star consists mainly of hydrogen, with an average of less than one atom of hydrogen for every 10,000 hydrogen atoms. Additionally, it contains approximately 1,000 helium atoms, 5 oxygen atoms, and various other elements.
There exist stars that exhibit an increase in their elemental composition. For instance, there are stars referred to as silicon stars, which possess a high concentration of silicon. Similarly, there are iron stars, manganese stars, carbon stars, and so on, which are characterized by an abundance of their respective elements.

Stars have a wide range of compositions when it comes to their elements. Young stars of the red giant variety tend to have higher levels of heavy elements. For example, one of these stars had an unusually high amount of molybdenum, 26 times more than what is found in the sun.
In general, as stars age, the amount of elements with atoms larger than a helium atom gradually decreases. However, the chemical composition of stars can also be influenced by their location in the galaxy.
Older stars
The spherical region of the galaxy is home to older stars that have a scarcity of heavy elements. These stars form a peripheral spiral arm, while the flat parts of the galaxy are responsible for the birth of new heavy stars. Therefore, the presence of heavy elements can be linked to the unique chemical evolution that characterizes the life of stars.
The chemical makeup of a star is a reflection of the interstellar medium and is influenced by two factors: nuclear reactions that occur during the star’s life cycle. Initially, a star’s composition is similar to the composition of the interstellar matter, which is the gas and dust cloud from which the star originated.
Gas-dust clouds exhibit variation in composition across different regions. For instance, stars formed in specific locations within the Universe are more prone to possess a higher abundance of heavier elements compared to stars that formed elsewhere.
When conducting spectral analysis to determine the composition of stars, numerous factors need to be considered, including gravity, temperature, and magnetic field. However, despite adhering to all the principles of the study, the collected data still appears to be incomplete. It is as if the inner workings of these remote entities remain elusive to investigation.
However, the composition of the stellar spectrum is the result of nuclear reactions (such as barium, technetium, and zirconium) that exhibit distinct indications of the presence of elements that can only be synthesized within the star’s core, resulting in a blending process of stellar matter. From the perspective of physicists, achieving equilibrium in the mixing of such vast amounts of stellar matter is exceedingly challenging, whereas for chemists, the process of nuclear reactions within a cosmic entity is exceptionally arduous.
An examination of globular star clusters within the Milky Way, which correspond to the oldest stars, has revealed a decrease in their composition. Conversely, if a galaxy predominantly originates from a hydrogen-containing gas cloud, it should also harbor stars composed solely of pure hydrogen.
Regarding the signs of transmutation that have altered the “chemical realm” of the star, these signs can occasionally be quite diverse. For instance, there are stars where hydrogen has potentially transformed into helium due to the mixing of the star’s substances, which has played a significant role in enriching the star (its outer layer) with helium.
Thus, A. A. Boyarchuk exclusively discovered stars with helium levels eight times higher than that of hydrogen, as well as only iron atoms per 1000 helium atoms in these stars. One of the helium stars contained no hydrogen whatsoever. This occurrence is uncommon and implies that the star’s hydrogen has been entirely consumed in the nuclear reaction. A thorough examination of one of these stars uncovered carbon, neon, and titanium.
There is another star made up of 500 carbon-0.56, nitrogen-0.72, oxygen-1.0, neon-3.2, silicon-0.05, and magnesium-0.5 helium atoms. This star is a bright double star in the Sagittarius constellation and has a surface temperature of approximately 10,000 ° C. Interestingly, this star lacks hydrogen and is very faint in its spectrum. However, it does have distinct helium lines. These stars are believed to have already burned out their hydrogen in a nuclear reaction. The presence of carbon and nitrogen in these stars provides valuable insights into the processes of nuclear reactions that power and create different elements.
Carbon stars are an intriguing phenomenon. These stars are characterized by their relatively low temperatures, as they are classified as giant and supergiant stars. The surface temperatures of these stars typically range from 2500 to 6000 ° C. At temperatures exceeding 3500 ° C, it has been observed that cyanide (C radical) and CH radicals, along with equal amounts of oxygen and carbon in the atmosphere, are present in these stars. These elements are derived from carbon compounds, such as carbon monoxide CO.
Additionally, titanium and zirconium oxide have been discovered in these stars, which possess the ability to withstand high temperatures. In instances where hydrogen is abundant, the concentrations of c, CO, and C2 are relatively low, while the concentration of CH increases. These types of stars, known as CH stars, coexist with stars that lack hydrogen.
There has been an observation of an increasing ratio of carbon to iron in a particular star. The carbon content was found to be 25 times higher than the iron content, while the ratio of carbon to hydrogen was 40. This suggests that the star is highly enriched in carbon and lacks a significant amount of hydrogen.
The variability in brightness of stars of this kind can also be attributed to a reduction in luminosity caused by the presence of solid carbon particles dispersed in the star’s atmosphere. However, it should be noted that most carbon stars do not possess the usual abundance of hydrogen, as indicated by the hydrogen content in their atmospheres.
Summary
Carbon stars possess a notable feature of having a high concentration of the carbon isotope 13C. This isotope plays a significant role in the star’s overall energy balance. The processes involving the participation of 13C occur at extremely high temperatures in the star’s deep region, providing it with energy. The presence of 13C in the star’s surface layer is likely a result of the mixing process.
Certain types of stars exhibit an increase in zirconium and metals found in the same group of the periodic table. These stars are formed either through neutron capture by the unstable technetium-4399c element in the technetium nucleus or during the photographic process from 98mo to 97mo. The presence of unstable nuclei strongly indicates the occurrence of nuclear reactions within stars.
A significant amount of research has been conducted by astronomers and astrophysicists in the field of spectral data, where they have analyzed and compared findings from meteorite studies. The conclusion drawn is that elements with even atomic numbers appear more frequently than those with odd atomic numbers. The stability of a nucleus is influenced by the proportion of protons to neutrons within it. Nuclei that formed under extreme conditions are more likely to be stable and have a higher chance of survival.
Feel free to send your assignments at any time of the day or night to ➔
For more information, you can visit the official website of Natalia Valeryevna Brilyonova, a teacher at the Department of Informatics and Electronics at Yekaterinburg State Institute.
All rights reserved by the copyright holders of the materials posted on this website. The use of these materials for commercial purposes or any other use, except for preliminary familiarization, is strictly prohibited. The publication and distribution of these materials is not intended for commercial gain.
This website is designed to assist full-time and part-time students in their educational journey. Natalia Brilyonova does not offer or provide any products or services.
If you wish to copy any materials, it is mandatory to include a web-link to natalibrilenova.ru.
© “Natalia Brilenova”.
When I was growing up in Siberia, the winter nights were incredibly dark, allowing the stars and moon to shine with a bright intensity. As a young child, I would often find myself standing beneath the night sky, mesmerized by the vast expanse of stars and constellations. This early fascination with the cosmos has stayed with me throughout my life, and I would love to share with you some intriguing facts about these celestial bodies.

Structure of a star
A star is composed of gas, making it unique among all celestial objects for its ability to emit light. Initially, cosmic dust and gases accumulate in a cloud known as a nebula. Over time, these materials blend together and eventually merge to form a singular celestial body. The interior temperature rises to millions of degrees, triggering a thermonuclear reaction that gives rise to a new, hot star.
Understanding the Composition of Stars
For many years, scientists were puzzled about the makeup of stars. However, a breakthrough method emerged that allowed them to uncover the answers to this fundamental question. Through the use of spectral analysis, scientists have developed the ability to determine the composition of stars. Atoms have the ability to emit and absorb light at various frequencies. By studying spectra, scientists can observe the distinct light and dark colors associated with each substance. It was through spectral analysis in the 19th century that it was revealed that stars are primarily composed of hydrogen and helium, along with trace amounts of heavier elements.

Are all stars made up of the same elements?
Interestingly, while the chemical makeup of stars may be similar, the proportions of the various elements can vary. This distinction allows stars to be classified into different categories based on their composition:

- Stars of the first generation. These stars have cores consisting only of helium. They emerged following the occurrence of the Big Bang.
- Stars of the second generation. These stars exhibit a low proportion of heavy metals.
- Stars of the third generation. This classification of stars possesses a greater proportion of heavy metals.
What is the makeup of the Sun
The Sun, similar to all other stars, is primarily composed of hydrogen and helium. In terms of its composition of heavier elements, it falls into the category of third-generation stars.

The Sun is the sole star positioned at an optimal distance from our planet, possessing an ideal chemical structure to sustain life on Earth.
Stars remain the most enigmatic and least examined entities. Our understanding of stars is built upon conjecture, as no human has ever achieved the feat of reaching one.

A celestial body known as the sun is an integral part of the vast expanse of the universe
The traditional understanding of the universe would be incomplete without the presence of stars, and one star in particular stands out – the Sun. Scholars have put forth the theory that the Sun is composed of a helium core, where hydrogen undergoes a chemical reaction to form helium, as well as a hydrogen layer.
It is widely accepted that the Sun has a diameter of approximately 1.4 million kilometers, which is 109 times greater than that of planet Earth. The surface temperature of this celestial body is estimated to be around 6,000 degrees Celsius.
Composition of a Star
A star is a massive luminous sphere composed primarily of hydrogen (70-73%) and helium (about 20-25%). It may also contain trace amounts of other chemical elements:
The emission of energy from nuclear reactions within the star generates its brilliant glow. Additionally, the outer layer of the star (approximately 2 kilometers thick) is a solid mixture of hydrogen and helium. The inner region of these celestial objects is thought to be in a liquid state, comprised of oxygen, neon, magnesium, sulfur, and silicon. Scientists postulate that the star’s core is primarily composed of iron.

Star enchantment
Throughout history, stars have been attributed with mystical significance. The stars have been used to predict the future, and they have served as guides for travelers navigating through unfamiliar territories. Legends and folklore are replete with mentions of stars and constellations:
- Ursa Major and Ursa Minor (Big Dipper and Little Dipper);
- Orion;
- the constellations of the zodiac (Taurus, Aquarius, Leo, Capricorn, etc.).
There is a captivating tale from ancient Greece about a woman named Callisto who experienced a forbidden love with Zeus, resulting in the birth of their son Arcades. In a fit of jealousy, Hera, Zeus’ wife, transformed Callisto into a bear, causing Arcades to unknowingly come close to killing his own mother. To protect Callisto and preserve their bond, Zeus elevated her to the heavens, turning her into the prominent constellation known as the Big Dipper. Additionally, Zeus transformed Arcades into the constellation Volopassus, a symbol of his eternal guardianship over his mother.
Given my extensive knowledge of the stars, I feel inclined to share some fascinating insights on this topic. I hope to provide an engaging and accessible exploration of this captivating subject.
Composition of Stars: A Unique Combination
When stars come into existence, they are composed of a specific set of elements in precise proportions. This holds true for all stars, whether they are massive giants or our comparatively “small” Sun. The fundamental components found within every star are:
What makes this even more fascinating is that, apart from a mere 2%, the Universe had the same exact composition at the moment of its explosive birth. The remaining matter gradually formed after the initial stellar explosions. Surprisingly, it is this 2% and its unique composition that accounts for the diverse range of star types we observe. Let me try to elucidate why this phenomenon occurs.
Exploring the chemical composition of stars

The role played by the 2%, which I have emphasized, is significant in this context. These particles have the ability to penetrate deep into the star, thereby reducing the mass threshold at which the star undergoes ignition. In other words, the presence of heavier matter leads to a faster ignition process.
In the popular imagination, the cosmos is often seen as a cold and empty place (as reflected in the song “Here the cold is cosmic, the color of the sky is different”). However, starting from the mid-19th century, scientists began to realize that the space between stars is not completely devoid of matter. One clear indication of the existence of interstellar matter is the presence of dark clouds, shapeless black patches that are particularly visible in the bright band of the Milky Way. In the 18th and 19th centuries, it was believed that these were actual “holes” in the distribution of stars. However, by the 1920s, it was understood that these spots are actually massive clouds of interstellar dust, which obstruct our view of the stars behind them (photo 1).

Image 1. The B69 dark nebula is a component of a massive interstellar dust cloud located in the Serpentine constellation. Picture sourced from www.eso.org
During the mid-19th century, a revolutionary period in the field of astronomy commenced when Gustav Kirchhoff and Robert Bunsen introduced spectral analysis. This groundbreaking method enabled scientists to identify the chemical makeup and physical characteristics of gases found in celestial objects. Astronomers swiftly recognized the immense potential of this new technique, leading to a surge of advancements in stellar spectroscopy throughout the 1860s. Concurrently, William Heggins, a remarkable observer, played a significant role in accumulating evidence for the existence of gases not only within stars but also in the vast expanse between them.
To detect the intrinsic brightness of interstellar gas in the visible spectrum, it must be both hot and dense. However, not all interstellar matter satisfies these criteria. Johannes Hartmann observed in 1904 that cooler and/or thinner interstellar gas can still be detected by the absorption lines it creates in stellar spectra. These absorption lines originate outside the star’s atmosphere, as the light travels from the star to the observer.
By the 1930s, the analysis of the emission and absorption lines of gas found in interstellar space had advanced significantly, allowing for a thorough examination of its chemical makeup. Through this study, it was determined that interstellar gas contains the same elements as those found on Earth. However, there were a few lines in the spectra that remained unidentified for a considerable period of time. A scientist named Heggins proposed that these unidentified lines indicated the presence of a new chemical element called nebulium (derived from the Latin word nebula, meaning cloud). However, further investigation revealed that these unidentified lines were actually caused by doubly ionized oxygen.
By the early 1930s, scientists believed that they had identified and assigned all the lines in the spectrum of interstellar gas to specific atoms and ions. However, in 1934, Paul Merrill made a discovery that challenged this belief. He reported the presence of four unidentified lines in the yellow and red regions of the spectrum. Unlike the previously observed interstellar lines, which were narrow and well-defined, these lines appeared broader and less distinct. This led scientists to hypothesize that they were not caused by atoms or ions, but by molecules. The question then became: what type of molecules could produce these lines? Some scientists speculated that they could be exotic molecules, such as sodium (Na2), or familiar two-atom compounds like the molecule CN, which had been discovered in comet tails by Heggins in the 19th century. It wasn’t until the late 1930s that the existence of interstellar molecules was confirmed. Several unidentified lines in the blue region of the spectrum were found to be associated with the compounds CH, CH+, and CN, providing definitive evidence for the presence of molecules in interstellar space.
The unique feature of chemical reactions in the interstellar medium is the prevalence of two-particle processes, where stoichiometric coefficients are always equal to one. Initially, it was believed that the only way to form molecules was through “radiative association” reactions, where two atoms collide and combine into a molecule by dissipating excess energy. If the molecule, formed in an excited state, manages to emit a photon before disintegration and transition to an unexcited state, it remains stable. Calculations conducted prior to the 1950s indicated that the observed abundance of these three simple molecules could be explained by assuming they are formed through radiative association reactions and destroyed by the interstellar radiation field, which is the combined radiation field from the stars in the Galaxy.
The scope of astrochemistry research was relatively limited in the early days, especially when it came to studying the interstellar medium. Scientists were only aware of three molecules and a few reactions involving them and their constituent elements. However, in 1951, David Bates and Lyman Spitzer made a groundbreaking discovery that changed everything. They reevaluated the equilibrium concentrations of molecules based on new data about the rates of radiative association reactions. This revealed that the formation of molecules from atoms occurred at a much slower rate than previously believed. As a result, the simple model used at the time greatly underestimated the presence of CH and CH+ by several orders of magnitude. Bates and Spitzer proposed an alternative explanation: these molecules weren’t formed from atoms but rather from the breakdown of more complex molecules, specifically methane. So where did the methane come from? It’s possible that it originated in stellar atmospheres and eventually made its way into the interstellar medium through dust particles.
Characteristics of the interstellar medium
When the first molecules were discovered in the interstellar medium, the physical properties and chemical composition were not well understood. The detection of CH and CH + molecules in the late 1930s provided important evidence for the presence of carbon and hydrogen. However, in 1951, the emission of interstellar atomic hydrogen at a wavelength of approximately 21 centimeters was detected, changing our understanding of the interstellar medium. It became clear that hydrogen is the most abundant element in this medium. According to current theories, interstellar matter consists mainly of hydrogen and helium, with heavier elements making up only 2% of the total mass. A significant portion of these heavier elements, particularly metals, can be found in dust particles. The total mass of interstellar matter in our Galaxy’s disk is several billion solar masses, accounting for about 1-2% of the disk’s total mass. In comparison, the mass of dust is around a hundred times less than the mass of gas.
The distribution of matter in interstellar space is not uniform. It can be categorized into three distinct phases: hot, warm, and cold. The hot phase consists of highly rarefied coronal gas, composed of ionized hydrogen with temperatures reaching millions of kelvins. This phase occupies approximately half of the galactic disk volume, with a density of around 0.001 cm-3. The warm phase, accounting for the other half of the disk volume, has a density of about 0.1 cm-3 and temperatures ranging from 8000 to 10,000 K. Hydrogen in this phase can exist in both ionized and neutral states. On the other hand, the cold phase is characterized by extremely low temperatures, not exceeding 100 K. In the densest regions, temperatures can drop to near absolute zero. Although the cold phase occupies only a small percentage of the disk volume, its mass accounts for about half of the total interstellar matter. Consequently, it has a relatively high density, with hundreds of particles per cubic centimeter or even more. While this density is significant in interstellar terms, it is considered a remarkable vacuum, measuring 10-14 torr, for electronic instruments.
The solid cold neutral gas possesses a delicate cloud structure, which can also be attributed to interstellar dust clouds. It can be inferred that dust clouds and gas clouds are essentially the same, with a combination of dust and gas. However, observations have indicated that the areas of space where the absorbing impact of dust is at its peak do not align with the regions of highest intensity of atomic hydrogen radiation. In 1955, Bart Bock and his colleagues proposed that in the most compact regions of interstellar clouds, particularly those that become opaque in the optical spectrum due to the dense dust concentration, hydrogen exists not in its atomic form, but rather in its molecular state.
Due to the fact that hydrogen constitutes the primary component of the interstellar medium, the designations of the various stages mirror the hydrogen’s state. An ionized medium signifies a state in which hydrogen is ionized, while other atoms may remain in a neutral state. Conversely, a neutral medium refers to a state in which hydrogen is neutral, albeit other atoms may be ionized. Dense, compact clouds, believed to be primarily composed of molecular hydrogen, are referred to as molecular clouds. It is within these clouds that the fascinating narrative of interstellar astrochemistry commences.
The emission of molecules occurs as a result of their additional degrees of freedom. A molecule possesses the ability to rotate, vibrate, and undergo more complex motions, each of which corresponds to a distinct set of energy levels. Similar to atoms, molecules absorb and emit photons when transitioning between these energy levels. Due to the relatively low energy associated with these motions, molecules can become easily excited even at low temperatures within molecular clouds. However, the photons emitted during these transitions do not fall within the visible range, but rather within the infrared, submillimeter, millimeter, and centimeter ranges. Consequently, the study of molecular radiation only gained traction once astronomers had access to instruments capable of observing in these long-wavelength ranges.
Nevertheless, the initial extraterrestrial molecule, which was identified through radio range observations, was still detected through absorption: back in 1963, it was observed in the radio emission of the supernova remnant Cassiopeia A. Specifically, this was the absorption line of hydroxyl (OH) with a wavelength of 18 cm, and shortly after that, hydroxyl was also detected through emission. In 1968, the emission line of ammonia was observed at a wavelength of 1.25 cm, and a few months later, water was discovered, with a line at 1.35 cm. A highly significant breakthrough in the exploration of the molecular interstellar medium occurred in 1970 with the identification of carbon monoxide (CO) emission at a wavelength of 2.6 mm.
Before that time, molecular clouds were somewhat theoretical objects. The most abundant chemical compound in the Universe, hydrogen molecule (H2), lacks transitions in the long wavelength area of the spectrum. In a molecular environment at low temperatures, it doesn’t emit light, meaning it remains invisible despite its high concentration. However, the H2 molecule does have absorption lines, but they are in the ultraviolet range, which can’t be observed from Earth’s surface. Observations require telescopes on high-altitude rockets or spacecraft, making them more complex and costly. With a subatmospheric instrument, the absorption lines of molecular hydrogen can only be observed in the presence of background stars. Considering that there aren’t many stars or celestial objects emitting in the ultraviolet range, and the fact that dust absorption is at its maximum in this range, it is clear that studying molecular hydrogen through absorption lines is very limited.

The usage of carbon monoxide (CO) as an indicator of molecular gas presence makes it a key component in mapping molecular gas distribution in the Galaxy. While some may question the wide utilization of CO, there is currently no suitable alternative. Therefore, caution must be exercised when interpreting CO observations to account for potential uncertainties.
Novel Approaches to Astrochemistry
In the early 1970s, the count of identified interstellar molecules started to escalate significantly. With each new discovery, it became evident that the existing chemical models, which only accounted for the presence of the first three molecules (CH, CH+, and CN), were not able to confidently explain the findings. As the number of identified molecules continued to rise, it became clear that these models were insufficient. In 1973, a fresh perspective on the chemical evolution of molecular clouds was introduced by William Watson and independently by Eric Herbst and William Klemperer, which is still widely accepted today.
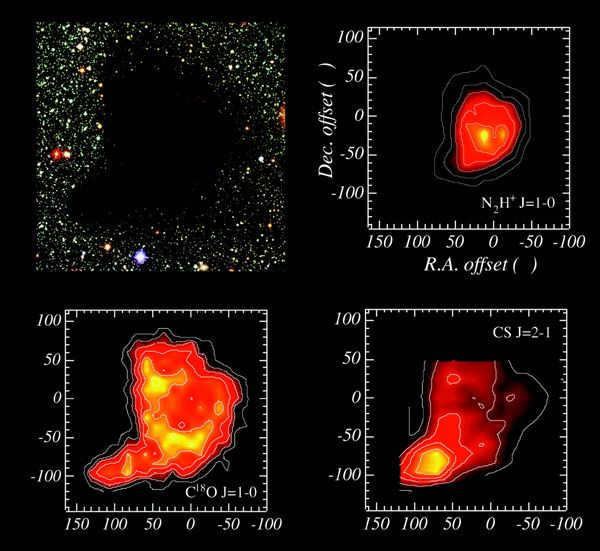
Located at the top left corner – the B68 globule is visible as a dark spot. This is caused by the dust within the globule, which blocks the light from background stars. Positioned above on the right – A map of the same globule shows the emission of N2H+.
The emission from this ion is confined to the central region of the globule. Displayed at the bottom left: the emission from the CO molecule forms an almost complete ring around the center of the globule. Presented at the bottom right: the emission from the CS molecule is only observed in a small clump at the edge of the globule.
Reference: Ch.J. Lada et al, Astrophysical Journal, 2003,586,286-295, doi:10.1086/367610
Watson, Herbst, and Klemperer proposed that ion-molecular reactions, which involve the interaction between neutral and ionized components, rather than radiative association reactions, play a crucial role in the formation of the molecular composition of frigid interstellar clouds. These reactions are not affected by temperature and, in some cases, may even become more prominent at low temperatures.
The issue at hand is that the ionization of the cloud’s substance needs to be limited. Radiation, whether it be from nearby stars or the collective radiation emitted by all the stars in the Milky Way galaxy, does not primarily ionize but rather dissociates. Furthermore, due to the presence of dust, radiation cannot penetrate deep into molecular clouds and only illuminates their outer regions.
However, there exists an additional ionizing element within the Galaxy called cosmic rays, which consist of atomic nuclei that have been accelerated to extremely high speeds through an unknown process. While the exact nature of this process remains uncertain, it is believed that the acceleration of cosmic rays, particularly those of interest to astrochemistry, occurs within the shock waves produced by supernova explosions. Similar to other forms of galactic matter, cosmic rays are primarily composed of fully ionized hydrogen and helium, specifically protons and alpha particles.
When H2 encounters the most abundant molecule, it ionizes it and transforms it into an H2+ ion. This ion then reacts with another H molecule to produce the H3+ ion. The H3+ ion plays a crucial role in all subsequent chemical reactions, as it enters into ion-molecular reactions with oxygen, carbon, and nitrogen. This process follows a general scheme, with the reaction involving oxygen looking like this:
The final step in this series of reactions is the dissociative recombination reaction between the hydroxonium ion and a free electron. This reaction produces a molecule that is fully saturated with hydrogen, such as water or hydroxyl. It is important to note that dissociative recombination can also occur with intermediate ions. As a result of these reactions, heavy elements can form water, methane, and ammonia. Another possibility is that a particle ionizes an atom of an impurity element (oxygen, carbon, nitrogen), and this ion then reacts with a molecule of H2 to form ions such as OH+, CH+, and NH+, which can further react with other ions. It is worth mentioning that chains of different elements do not occur in isolation; their intermediate components react with each other. As a result of this “cross-pollination,” most of the carbon is converted into CO molecules, while the remaining unbound oxygen forms water and O2 molecules. The N molecule becomes the main reservoir of nitrogen2. Any atoms that are not part of these basic components become part of more complex molecules, with the largest known molecule consisting of 13 atoms.
Nevertheless, there exists a more significant instance: the absence of molecular hydrogen formation in the gaseous state! The mechanism involving ion-molecular reactions functions solely in the presence of preexisting H molecules within the medium2. However, their origin remains a mystery. Despite the existence of three potential approaches to generate molecular hydrogen in the gaseous phase, all of them exhibit an exceptionally sluggish pace and are incapable of operating in galactic molecular clouds. The resolution to this predicament lies in revisiting one of the preceding mechanisms, specifically reactions occurring on the surfaces of cosmic dust particles.
Just like previously, the dust particle in this mechanism acts as the third entity, creating favorable conditions on its surface for the combination of atoms that are unable to unite in the gaseous state. In a low-temperature setting, loose hydrogen atoms adhere to the dust particles, but due to thermal fluctuations, they do not stay in one location and instead move around on the particle’s surface. During these wanderings, two hydrogen atoms can come together and form a molecule H2. The energy released during this reaction causes the molecule to detach from the dust particle and enter the gas phase.
If a hydrogen atom encounters a different atom or molecule on the surface, the resulting reaction will be altered. However, it is worth considering whether there are other substances present on the dust. Modern observations of the densest sections of molecular clouds, known as nuclei, suggest that there are. These nuclei may eventually evolve into stars with planetary systems. Within the nuclei, chemical differentiation occurs. The densest part of the nucleus primarily emits radiation from nitrogen compounds such as ammonia and N2H+. On the other hand, carbon compounds like CO, CS, and C2S emit light in the surrounding shell of the nucleus. As a result, radio emission maps depict these nuclei as compact spots of nitrogen compound emission surrounded by rings of carbon oxide emission.
The contemporary explanation for differentiation is as follows: within the densest and coldest region of the molecular core, carbon compounds, primarily CO, solidify into particles of dust, forming icy mantles on them. In the gas phase, these compounds are only preserved at the outer edges of the core, where it is possible that radiation from the stars of the Galaxy penetrates and partially vaporizes the icy mantles. The situation with nitrogen compounds is different: the primary molecule containing nitrogen, N2, does not solidify into dust as quickly as CO, allowing enough nitrogen to remain in the gas phase even within the coldest portion of the core for a longer period of time, thus producing the observed quantities of ammonia and N2H+.
Chemical reactions are occurring in the frozen layers of dust mantles, with the main process being the addition of hydrogen atoms to the molecules. An example of this is the step-by-step addition of H atoms to CO molecules in the icy mantles, resulting in the creation of methanol. More intricate reactions that involve components other than hydrogen lead to the formation of various multi-atomic molecules. When a young star ignites within the core, its radiation causes the dust particle mantles to vaporize, releasing the chemically synthesized products into the gas phase, allowing for observation.
Triumphs and obstacles
All current information on chemical reactions in the interstellar and circumstellar medium is compiled in specialized databases, with two of the most popular being UDFA (UMIST Database for Astrochemistry) and KIDA (Kinetic Database for Astrochemistry).
These databases serve as comprehensive collections of reactions involving two reactants, multiple products, and numerical parameters (ranging from one to three) that allow for the calculation of reaction rates based on temperature, radiation field, and cosmic ray flux. While there is less standardization when it comes to reactions occurring on dust particle surfaces, there are still two or three commonly used variants that are prevalent in most studies of astrochemistry. By utilizing the reactions included in these databases, it becomes possible to quantitatively explain the molecular composition observations of objects of varying ages and physical conditions.
Astrochemistry is currently progressing in four different areas.
There is currently a lot of interest in the chemistry of isotopomers, specifically the chemistry of deuterium compounds. Alongside hydrogen atoms, there are also deuterium atoms present in the interstellar medium, in a ratio of about 1:100,000, which is similar to the presence of other impurity atoms. In addition to hydrogen molecules, HD molecules are also formed on the dust particles. In colder environments, the reaction
H3 + + HD → H2D + + H2
is not balanced by the reverse process. The H ion2D + plays a similar role in chemistry to the H ion3 + , and this allows deuterium atoms to spread to more complex compounds. The result is quite fascinating: with a general D/H ratio of around 10 -5, the ratio of deuterated molecules to their non-deuterated counterparts (e.g., HDCO to H2CO, HDO to H2O) can reach percentages or even tens of percent. Another area of improvement in modeling is to consider differences in the chemistry of carbon and nitrogen isotopes.
Another important area of astrochemical research focuses on reactions that occur on the surfaces of dust particles. Much work is currently being conducted to understand how these reactions are influenced by the properties and temperature of the dust particle surface. However, there is still much to learn about the evaporation process of organic molecules that are synthesized on these dust particles.
In addition, chemical models are increasingly being used to study the dynamics of the interstellar medium, including the formation of stars and planets. This is crucial because it enables researchers to directly correlate numerical descriptions of matter movement in the interstellar medium with observations of molecular spectral lines. Furthermore, this research also has astrobiological implications as it relates to the potential for interstellar organic matter to reach newly forming planets.